Abstract
Background: It is important to identify inherited bone marrow failure syndromes (IBMFS) in patients with aplastic anemia in order to provide appropriate therapy. IBMFS are diagnosed through genetic or other diagnostic testing but can also go unrecognized, particularly in adults who may lack classic IBMFS features. This is particularly critical in light of increasing identification of individuals with variants of uncertain significance (VUS) and those with single pathogenic variants in an autosomal recessive (AR) gene or X-linked recessive (XLR) gene in females (SPVR) ("carriers") during evaluation. In this study of patients diagnosed with acquired severe aplastic anemia (SAA), we evaluated putatively causal variants in IBMFS genes to determine the frequency of patients with an unrecognized IBMFS or SPVR and assessed their association with outcomes after hematopoietic cell transplant (HCT).
Methods: We used pre-HCT blood samples and clinical data from the Transplant Outcomes in Aplastic Anemia study (TOAA; a collaboration between the National Cancer Institute and the Center for International Blood and Marrow Transplant Research). Germline whole exome sequencing was performed on 732 patients with acquired SAA who received HCT between 1991-2015. A total of 104 IBMFS genes, (51 autosomal dominant (AD), 46 AR, 3 both AR and AD, 4 XLR) were evaluated for both single nucleotide (SNV) and copy number variants (CNV). All variants were curated using ACMG/AMP criteria, and a subset were validated by Sanger sequencing. Variants classified as VUS according to ACMG/AMP criteria and with a damaging score prediction in 3 of 5 in silico meta-predictors were categorized as deleterious VUS. Patients were divided into 3 groups based on known inheritance patterns of identified genes into those with 1) unrecognized IBMFS 2) SPVR or 3) neither (presumed acquired SAA). For telomere biology genes with AD/AR inheritance, we used telomeres <10 th percentile for age (by qPCR) to distinguish IBMFS from SPVR. For statistical analysis, we used the Kaplan-Meier estimator to calculate the probability of overall survival. The log-rank test was used to compare the survival distribution across patient categories. Cox proportional hazard models were used for multivariable analysis.
Results: We identified 309 variants of them 156 were pathogenic or likely pathogenic (P/LP) (112 (71.8%) loss of function SNVs, and 10 CNVs), and 153 were deleterious VUS. Patients with deleterious VUS did not have different survival compared with those with no variants, and these were not considered for designating unrecognized IBMFS and SPVR cases
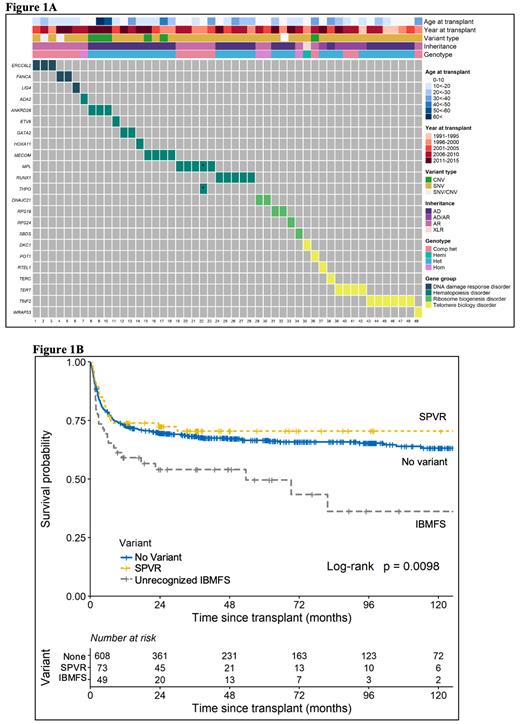
Our analysis showed that 6.7% (N=49/732) of patients had variants consistent with an unrecognized IBMFS; 22 were AR (21 compound heterozygous, 1 homozygous), 26 AD and 1 XLR. Approximately half of those patients (22/49, 45%) had P/LP variants in hematopoiesis genes, and 31% in telomere biology genes (Figure 1A). We identified 79 patients with an SPVR, most in SBDS and FANCM.
Patients with an unrecognized IBMFS had worse overall survival when compared with patients with presumed acquired SAA (log-rank p=0.0098) (Figure 1B). Multivariable analysis confirmed this association (HR=2.11, 95% confidence interval (CI)=1.38-3.22, p=0.001). The observed survival difference was not mitigated by lower conditioning regimen intensity (HR=2.1, p=0.01 in myeloablative condition (MAC), and HR=2.3, p=0.016, in reduced intensity regimens (RIC)).
Patients with an SPVR had no post-HCT survival difference than presumed acquired SAA regardless of conditioning regimen (overall HR=0.96, p=0.85; MAC HR=1.4, p=0.27; RIC HR=0.3, p=0.1).
Conclusions: A sizable subset of patients (6.7%) with reported immune mediated acquired SAA had unrecognized inherited disorder and 33% were adults at HCT. Unrecognized IBMFS was associated with statistically significant poorer survival after HCT. In contrast, post-HCT survival in patients with an SPVR ("carriers") was not different than those with acquired SAA. This work underscores the importance of identifying SAA patients with clinically meaningful underlying inherited disorders to enable the use therapeutic approaches to minimize regimen toxicity and late complications. It further highlights that identification of a single pathogenic variant in an autosomal recessive gene or X-linked gene in females and VUS are associated with post-HCT survival similar to acquired SAA.
Paczesny: Medical University of South Carolina: Patents & Royalties: inventor on the ST2 bispecific antibody patent application. Lee: Kadmon: Research Funding; National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; JANSSEN: Other; AstraZeneca: Research Funding; Takeda: Research Funding; Novartis: Other: clinical trials, Research Funding; Syndax: Research Funding; Pfizer: Research Funding; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal